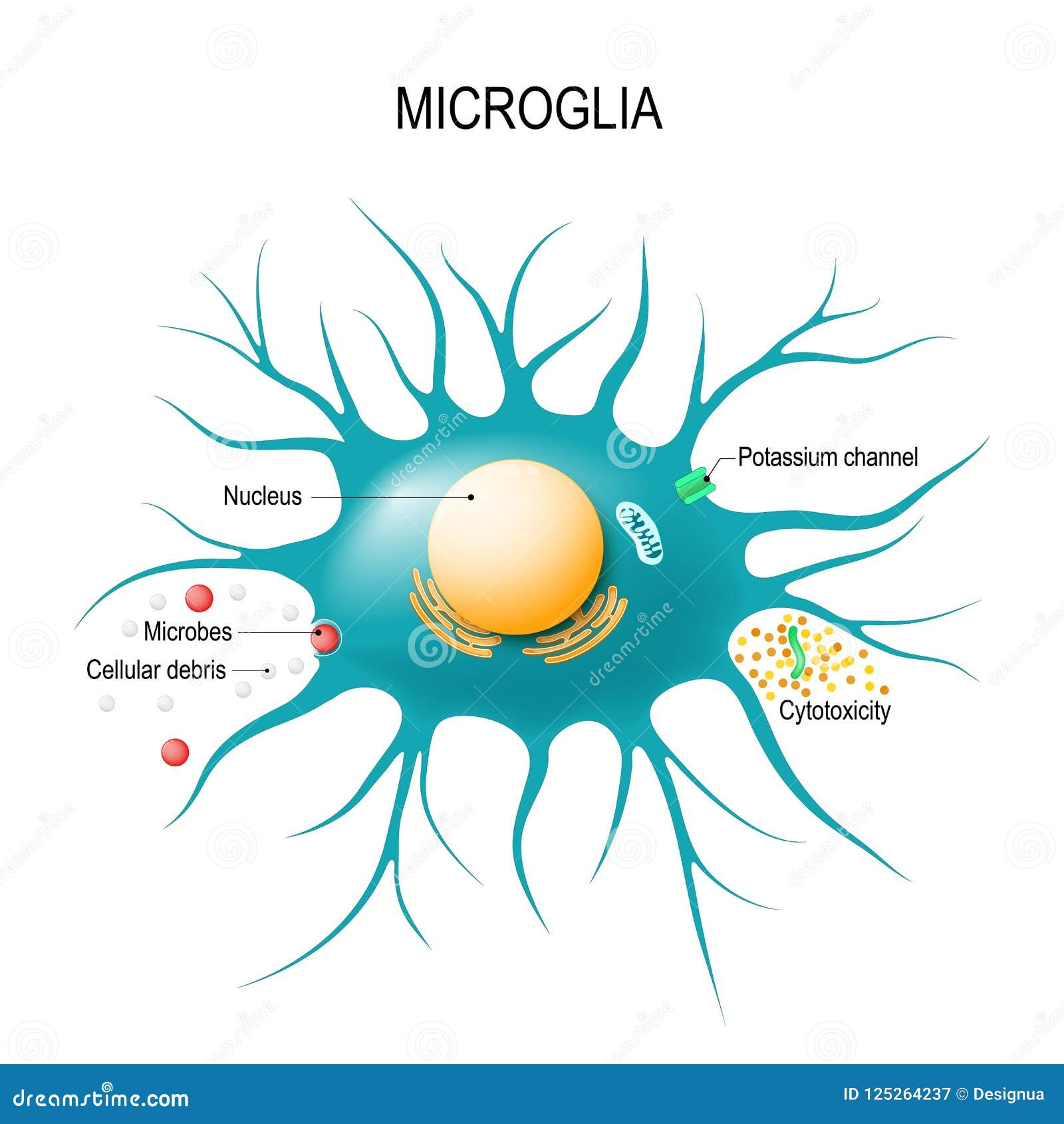
Microglial cells, the brain’s primary immune defenders, are at the forefront of a groundbreaking understanding of neurodegenerative diseases such as Alzheimer’s disease. These specialized cells play a crucial role in maintaining brain health by monitoring and responding to cellular damage, as well as performing essential tasks like synaptic pruning. Their ability to remove dead neurons and optimize synaptic connections ensures the brain functions properly and adapts to changes. Research led by Beth Stevens, a leading neuroscientist at the Broad Institute, highlights how dysfunctional microglial activity can contribute to the development of conditions like Alzheimer’s. By unveiling the complexities of these immune cells, Stevens’ work paves the way for innovative therapies and biomarkers aimed at combating the challenges of neurodegenerative diseases affecting millions of individuals.
Within the realm of the brain’s defenses, microglial cells—often referred to as the brain’s immune system—perform vital functions that are increasingly linked to neurological disorders. These glial cells act as custodians, protecting the brain from injury and infection while also engaging in the significant process of removing unnecessary connections between neurons. The studies conducted by researchers, including the notable Beth Stevens, have unearthed the dual nature of microglial activity, showcasing how beneficial functions can become detrimental, particularly in the context of Alzheimer’s disease and other cognitive decline conditions. As we delve deeper into their role, it becomes apparent that understanding these immune cells is essential for developing targeted treatments for neurodegenerative diseases. By identifying the intricate relationship between microglia and synaptic pruning, researchers are one step closer to unlocking new therapeutic avenues.
Microglial Cells: The Guardians of Brain Health
Microglial cells are often regarded as the brain’s immune sentinels, playing a critical role in maintaining overall brain health. These specialized glial cells constantly monitor the brain environment, looking for signs of damage or disease. When they detect dead or dysfunctional cells, microglia spring into action, performing essential tasks such as synaptic pruning—removing unnecessary or damaged synapses to ensure efficient neural communication. This regulatory function not only supports healthy brain development but also highlights the potential link between microglial activity and various neurodegenerative diseases, including Alzheimer’s disease.
Recent research has showcased how dysregulation of microglial activity can contribute to the pathogenesis of Alzheimer’s disease. In the brain of an Alzheimer’s patient, microglial cells may engage in excessive or insufficient pruning of synapses, leading to disrupted neural circuitry and cognitive decline. Understanding these processes is pivotal for developing targeted therapies that can enhance or restore proper microglial function, thereby slowing or preventing the progression of neurodegenerative diseases.
The Role of Synaptic Pruning in Neurodegenerative Disorders
Synaptic pruning is a fundamental process in brain development and functioning, primarily orchestrated by microglial cells. This process helps shape neural circuits by eliminating redundant or ineffective synapse connections, thus optimizing brain function. However, in the context of neurodegenerative diseases, abnormalities in synaptic pruning have been observed. As highlighted by the Stevens Lab, improper pruning can exacerbate the effects of conditions such as Alzheimer’s, resulting in the loss of critical neural connections that are vital for memory and cognitive performance.
Research into synaptic pruning has brought researchers closer to understanding the complexities of diseases like Alzheimer’s. By studying the mechanisms through which microglia prune synapses, scientists can identify pathways that may contribute to cognitive decline. This knowledge holds promise for developing innovative therapeutic strategies aimed at correcting synaptic pruning disorders, potentially reversing some of the cognitive deficits associated with Alzheimer’s disease and other neurodegenerative conditions.
Innovative Research: Beth Stevens and Her Impact
Beth Stevens, an acclaimed neuroscientist, has made significant contributions to the understanding of microglial cells and their role in neurodegenerative diseases. Supported by funding from the National Institutes of Health, Stevens has pursued groundbreaking research that implicates microglia in the synaptic pruning process, elucidating how these immune cells can both sustain and compromise brain health. Her insights have shaped the direction of research in Alzheimer’s and inspired numerous projects aimed at investigating the intricate relationship between the immune system and brain function.
Stevens’ work exemplifies how curiosity-driven science can pave the way for new breakthroughs in understanding diseases. Her recognition, including the prestigious MacArthur “genius” award, underscores her role not just as a researcher but as a pathfinder in an emerging field that intertwines neuroscience and immunology. By following the trail of scientific inquiry that started with simple observations of microglial behavior, Stevens illuminates the broader implications for treating Alzheimer’s and similar neurodegenerative diseases.
Neurodegenerative Diseases: Understanding the Underlying Mechanisms
Neurodegenerative diseases, such as Alzheimer’s and Huntington’s disease, pose a significant challenge to public health. These disorders are characterized by progressive neuronal loss and associated cognitive decline. The intricate interplay between genetic, environmental, and immunological factors complicates the understanding of these diseases. Central to these challenges is the role of microglial cells in facilitating or mitigating neuronal damage, which can provide clues to potential therapeutic interventions.
The study of neurodegenerative diseases emphasizes the need for comprehensive research into the mechanisms that drive these conditions. By examining how factors like synaptic pruning interplay with neuroinflammation mediated by microglia, new avenues for treatment can be explored. Understanding these mechanisms is critical not only for improving patient care but also for developing preventive strategies to reduce the incidence and impact of neurodegenerative disorders across populations.
Exploring Therapeutic Avenues Through Microglial Research
The research on microglial cells spearheaded by scientists like Beth Stevens opens doors to innovative therapeutic strategies for neurodegenerative diseases like Alzheimer’s. By elucidating the roles of microglia in synaptic pruning and neuroinflammation, researchers are better equipped to uncover targeted therapies that can enhance the immune response within the brain. This burgeoning field not only holds promise for treating Alzheimer’s but also for other disorders where microglial dysfunction is implicated.
As researchers develop new biomarkers based on microglial activity, clinicians may gain valuable tools for early diagnosis and intervention in neurodegenerative diseases. The potential for targeting microglial functions presents a transformative approach to managing Alzheimer’s disease, as enhancing or restoring normal microglial activity could lead to improved patient outcomes. Ongoing research in this arena continues to correlate microglial behavior with the underlying processes of neurodegeneration, ultimately striving for solutions that can revolutionize patient care.
The Impact of Early Research on Alzheimer’s Progression
The contributions of early foundational research in the field of Alzheimer’s continue to resonate throughout contemporary studies. Pioneers like Beth Stevens have forged the path for current investigations into the role of microglial cells and their influence on neurodegenerative diseases. Each discovery builds upon previous findings, enhancing the scientific community’s understanding of how the brain’s immune system interacts with neuronal health and dysfunction.
The lessons learned from early research not only highlight the complexities of the disease but also reinforce the significance of continued inquiry into the pathophysiological mechanisms of Alzheimer’s. As scientists continue to delve into the nuances of synaptic pruning and microglial activity, there is hope that varying therapeutic approaches will emerge, ultimately changing the trajectory of Alzheimer’s disease management and improving quality of life for millions.
Funding and Support: A Pillar of Neuroscience Research
The success of research in neurodegenerative diseases is largely contingent upon adequate funding and support from organizations such as the National Institutes of Health (NIH). As demonstrated in Beth Stevens’ journey, federal grants are instrumental in propelling groundbreaking research forward. Sustained funding allows researchers to explore novel hypotheses and to employ innovative methodologies that can yield critical insights into conditions like Alzheimer’s disease.
Moreover, support for basic science research lays the foundation for translational studies that move findings from the lab to clinical application. The interconnectedness of funding and scientific advancement highlights the importance of advocating for resources directed towards understanding neurodegenerative diseases. Communities, healthcare providers, and policymakers must unite to prioritize funding that fosters pioneering research, ultimately leading to enhanced strategies for detection and treatment of Alzheimer’s and related disorders.
The Importance of Understanding the Brain’s Immune System
Recognizing the role of the brain’s immune system, particularly through microglial cells, is pivotal in addressing the complexities of neurodegenerative diseases like Alzheimer’s. These immune cells not only fend off pathogens and debris but also play a crucial role in neural communication and homeostasis. Disruptions in microglial function can lead to adverse outcomes, making it essential for research to focus on how these cells interact with neurons and influence overall brain health.
Understanding the brain’s immune system can also shine a light on potential links between neuroinflammation and cognitive decline. As science evolves, it opens opportunities to explore how immune modulation can serve as a therapeutic target. Enhancing our grasp of microglial biology may yield new insights into how to tailor treatments that address the immune aspect of neurodegenerative diseases, potentially resulting in more effective interventions.
Bridging the Gap: From Animal Models to Human Applications
Research on microglial cells has primarily been conducted in animal models, particularly in mice, to study their functions and behaviors in a controlled setting. This approach has provided invaluable insights into how microglia behave under various conditions, including neurodegenerative diseases like Alzheimer’s. However, translating these findings from animal studies to human applications remains a significant challenge. Understanding the similarities and differences between species is crucial for ensuring successful therapeutic outcomes.
As scientists work to bridge the gap between animal models and human research, ongoing collaborations with clinicians and applied researchers can enhance the relevance of findings. Integrating discoveries related to microglial function into clinical practice will be key to developing treatments that directly address human disease processes. This collaborative approach, centered around a deep understanding of brain immune mechanisms, can ultimately lead to breakthroughs in the fight against neurodegenerative diseases.
Frequently Asked Questions
What is the role of microglial cells in Alzheimer’s disease?
Microglial cells act as the brain’s immune system and play a crucial role in managing Alzheimer’s disease. They monitor the brain for signs of illness, remove dead or damaged neurons, and prune synapses, which are essential for communication between neurons. Dysfunctional microglial activity can disrupt synaptic pruning and contribute to the neurodegenerative processes seen in Alzheimer’s.
How do microglial cells contribute to neurodegenerative diseases?
Microglial cells are vital for maintaining brain health; however, their improper functioning can lead to neurodegenerative diseases such as Alzheimer’s and Huntington’s disease. This occurs when these immune cells fail to effectively prune synapses or manage damaged cells, which may accelerate the degeneration of neuronal circuits and contribute to disease symptoms.
What is synaptic pruning and how is it related to microglial cells?
Synaptic pruning is a process mediated by microglial cells, where excess neuronal connections are eliminated to optimize brain function. In Alzheimer’s disease, faulty synaptic pruning by microglia can lead to disrupted neural circuits and cognitive decline. Research, including work by Beth Stevens, highlights the importance of proper synaptic pruning in maintaining cognitive health.
What recent discoveries about microglial cells have been made in relation to Alzheimer’s disease?
Recent discoveries by Beth Stevens and her lab have shown that microglial cells are involved in improper synaptic pruning, which may exacerbate Alzheimer’s disease. This research indicates that targeting microglial function could lead to innovative therapies and biomarkers for managing this and similar neurodegenerative diseases.
How can understanding microglial cells improve treatments for neurodegenerative diseases?
Understanding the role of microglial cells in neurodegenerative diseases can lead to innovative treatment strategies. Identifying how microglia improperly prune synapses or fail to clear damaged cells can help scientists develop therapies aimed at restoring normal microglial function, potentially alleviating symptoms of Alzheimer’s disease and other disorders.
Who is Beth Stevens and what is her contribution to microglial research?
Beth Stevens is a prominent neuroscientist known for her groundbreaking research on microglial cells and their role in neurodegenerative diseases such as Alzheimer’s. Her work has enhanced the understanding of how microglia function as part of the brain’s immune system, emphasizing their importance in synaptic pruning and overall brain health, paving the way for new treatment approaches.
What are some implications of recent research on microglial cells for Alzheimer’s disease?
Recent research on microglial cells has significant implications for Alzheimer’s disease, particularly in understanding how these cells can contribute to disease progression through improper synaptic pruning. This knowledge may lead to the development of new biomarkers and therapeutic strategies aimed at correcting microglial dysfunction, which could help manage symptoms and improve patient outcomes.
| Key Points | Details |
|---|---|
| Role of Microglial Cells | Act as the brain’s immune system, monitoring for illness and aiding in the removal of dead cells. |
| Impact on Neurodegenerative Diseases | Improper pruning of synapses by microglia contributes to Alzheimer’s, Huntington’s, and other disorders. |
| Research Foundation | Beth Stevens emphasizes the importance of foundational research supported by the NIH. |
| Achievements | Stevens received a MacArthur ‘genius’ award for her contributions to the understanding of microglial cells. |
| Clinical Relevance | Research may lead to new biomarkers and therapies, impacting the care of millions with Alzheimer’s. |
Summary
Microglial cells are crucial for maintaining brain health and play a significant role in neurodegenerative diseases. The groundbreaking research conducted by Beth Stevens highlights their function in synaptic pruning and how improper activity can lead to conditions like Alzheimer’s disease. Understanding microglial cells not only deepens our knowledge of the brain’s immune defense but also paves the way for potential treatments for millions suffering from these debilitating conditions.