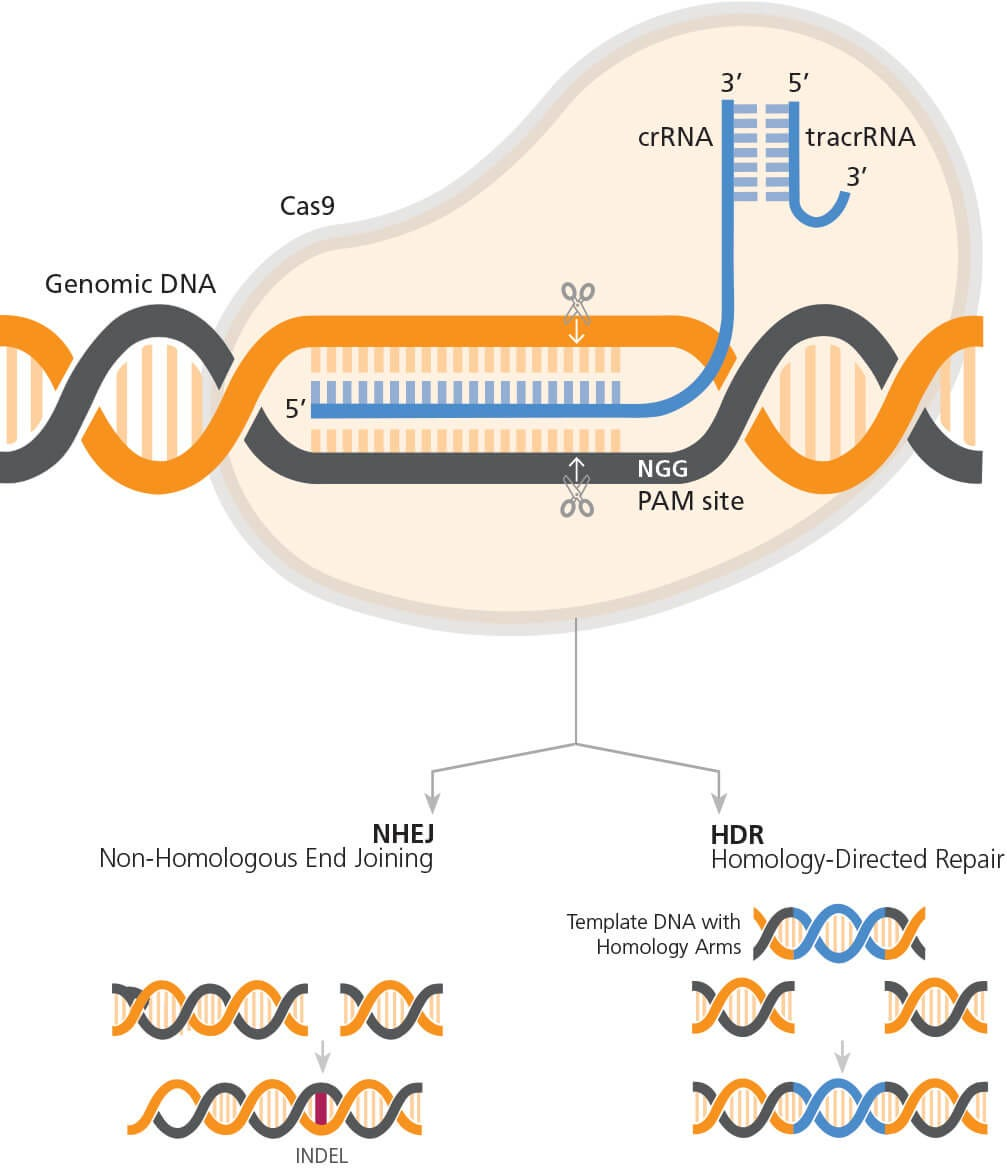
CRISPR gene editing is revolutionizing the landscape of biotechnology by offering unprecedented possibilities for precise genetic manipulation. This innovative tool allows scientists to edit genes with remarkable accuracy, opening the door to potential cures for genetic disorders, including sickle cell anemia. However, the advancement of such medical technology also brings forth critical discussions surrounding CRISPR ethics and the implications for health equity. As researchers explore the capabilities of gene editing, they must consider not only the benefits but also the ethical conundrums it presents. The balance of progress in gene editing with moral responsibility is a central theme that continues to shape the conversation in modern medicine.
The emergence of CRISPR technology has introduced a new era in the field of genetic modification, often referred to as gene editing. This groundbreaking advancement permits targeted alterations to DNA, enabling the possibility of eradicating genetic ailments like sickle cell anemia. Yet, as we explore these cutting-edge medical technologies, we confront significant questions regarding the ethics of such interventions. The balance of innovation and responsibility raises issues of justice in health, particularly in terms of equitable access to these treatments. Ultimately, the decision to harness the power of genetic editing demands careful consideration of the social and ethical implications that arise.
The Promise of CRISPR Gene Editing in Medicine
CRISPR gene editing has revolutionized the field of medical technology, offering unprecedented opportunities for curing genetic disorders. The ability to precisely edit genes at specific locations holds promise for conditions like sickle cell anemia, where recent advancements have shown that somatic cell modification can effectively eliminate the disease in patients. This innovation opens the door to potentially eradicating other genetic disorders, presenting a powerful tool for healthcare professionals in the quest for effective treatments.
However, the promise of CRISPR gene editing also brings forth significant ethical dilemmas. While the prospect of curing life-threatening diseases is appealing, it raises questions about the extent to which we should alter human genetics. Should gene editing be reserved for severe conditions, or are there cases where modifying traits deemed non-pathological, such as those related to disability, could be ethically justified? The discussion is ongoing among scientists, ethicists, and the public, emphasizing the need for a careful examination of these capabilities.
Ethical Implications of CRISPR in Treating Sickle Cell Anemia
The treatment of sickle cell anemia through CRISPR technology exemplifies the ethical considerations that accompany powerful medical interventions. Neal Baer’s personal experiences with children suffering from this debilitating condition illustrate the urgent need for effective solutions, yet they also prompt a critical look at who benefits from such advancements. The high cost of gene editing therapies, like the estimated $2.2 million for sickle cell treatment, raises important questions regarding health equity—specifically, who can access these life-changing treatments.
In discussions surrounding CRISPR and sickle cell, it is essential to consider health equity implications. Access to groundbreaking medical treatments has historically been skewed towards those with better socioeconomic status, meaning that many patients in lower-income brackets may be excluded from benefiting from advanced therapies. As healthcare systems evolve and gene editing becomes commercially available, it’s crucial for policymakers and practitioners to consider frameworks that ensure equitable distribution of such technologies, addressing the disparity often evident in modern medicine.
Addressing Health Equity in Gene Editing Approaches
Health equity is a pivotal theme in the discourse on gene editing technologies. As CRISPR becomes more integrated into clinical practice, it is imperative to confront the socio-economic barriers that prevent marginalized communities from accessing these advancements. With an estimated 100,000 individuals affected by sickle cell anemia in the U.S., the disparity in treatment availability underscores the need for systemic changes that promote fair access to innovative therapies.
Moreover, discussions around health equity in gene editing must also consider the ethical dimensions of choice and consent. For instance, if parents opt for genetic modifications based on perceived sociocultural advantages, such as eliminating traits they consider undesirable, it raises the question of whether the interests of the child are genuinely prioritized. A balanced approach that respects individual choice while safeguarding against potential societal pressures is essential to ensure that gene editing technologies promote justice and fairness across all demographics.
The Intersection of CRISPR Ethics and Medical Technology
As medical technology continues to evolve, the ethics surrounding CRISPR gene editing remain at the forefront of scientific debates. The capacity to alter genetic makeups introduces complex moral considerations, particularly when addressing non-fatal conditions that might simply reflect human diversity rather than pathology. This raises profound questions on the nature of humanity: Should we intervene in our genetic destiny, and if so, to what extent? Such discussions are vital in shaping a responsible path forward in biomedicine.
Additionally, the ethical landscape of CRISPR applications necessitates robust oversight and international guidelines to prevent misuse. The potential for genetic manipulation to extend beyond therapeutic applications into areas like human enhancement or military use highlights the urgent need for global dialogue on ethical standards. Stakeholders in the field—scientists, ethicists, and regulators—must work collaboratively to establish frameworks that balance innovation with ethical responsibility, ensuring that the utilization of CRISPR technology serves the greater good.
Unintended Consequences of Gene Editing Technologies
While CRISPR technology offers exciting possibilities, scientists express concern about unintended consequences that could arise from gene manipulation. The case of LDL cholesterol gene editing illustrates this, as altering a gene not only impacts cholesterol levels but also affects various other physiological processes. The complexities of genetic interactions suggest that changing one aspect of an organism’s genome may have ripple effects that are not yet fully understood, making thorough research and ethical consideration paramount before proceeding with widespread applications.
Moreover, evolving our understanding of genetics through CRISPR necessitates a cautious approach to clinical trials and patient applications. As evidenced in the case of genetic modifications aimed at curing or preventing diseases, each intervention could lead to unforeseen repercussions that challenge our comprehension of genomic stability and human health. Emphasizing the need for comprehensive studies and ongoing monitoring can help mitigate potential risks and pave a safer pathway for advancing gene editing technologies.
The Role of Media in Shaping Perceptions of Gene Editing
The media plays a crucial role in shaping public perception and understanding of gene editing technologies like CRISPR. Through the dissemination of information via documentaries, news segments, and digital content, media influences how society perceives the ethics, possibilities, and risks associated with gene editing. Engaging narratives can highlight both the potential benefits for health innovations, like curing sickle cell anemia, while also ensuring that audiences are aware of the ethical complexities involved.
Furthermore, the portrayal of gene editing in popular culture, notably through television dramas, can spark dialogue about the implications of such technologies. Writers, like Neal Baer, often weave real-life medical debates into entertaining narratives, making complex scientific concepts more relatable. This fusion of storytelling with bioethics can foster a broader public discourse, encouraging informed discussions that impact policy and personal viewpoints about CRISPR and its role in the future of medicine.
Potential Benefits of CRISPR Beyond Disease Treatment
Though much of the current focus on CRISPR technology revolves around disease treatment, its potential benefits extend beyond medical applications. By enabling scientists to explore genetic variations, CRISPR may contribute to advancements in agricultural biotechnology, thus improving food security and sustainability. The ability to enhance crop resilience against pests and adverse climates could reshape how communities approach food resources, addressing both environmental and societal challenges.
Moreover, as researchers continue to uncover the broader applications of CRISPR, industries such as environmental management and conservation may benefit from genetic editing’s precision. For example, deploying CRISPR technology in endangered species could assist in genetic diversity restoration efforts, reinforcing ecological balance. By recognizing the multi-faceted nature of gene editing, stakeholders can advocate for responsible innovation that promotes both public health and environmental welfare.
Legislation and Oversight in CRISPR Applications
The implementation of CRISPR technology into clinical practice highlights the urgent need for comprehensive legislation and oversight. As the technology is capable of profound genetic changes, establishing regulatory frameworks will help ensure its ethical application while safeguarding public health. Policymakers are tasked with addressing potential risks, including gene editing’s implications for human rights and genetic discrimination, in order to create a responsible pathway for utilization in medicine and beyond.
Additionally, international cooperation is essential to tackle the global challenges posed by gene editing technologies. The uneven landscape of regulatory standards in different countries may lead to ethical dilemmas, where individuals seek treatments in less regulated environments. Collaborative initiatives between governments, bioethics committees, and scientific communities are vital to shaping cohesive guidelines that can govern CRISPR applications, thus aligning public health objectives with ethical standards.
Frequently Asked Questions
What are the main applications of CRISPR gene editing in medicine?
CRISPR gene editing is primarily used for correcting genetic defects, treating diseases like sickle cell anemia, and advancing research on genetic conditions. By allowing scientists to edit specific genes, CRISPR can potentially cure hereditary diseases by either repairing defective genes or altering genes responsible for disease development.
How does CRISPR gene editing offer a cure for sickle cell anemia?
CRISPR gene editing provides a revolutionary treatment for sickle cell anemia by precisely editing the genes responsible for the disease in patients’ somatic cells. This manipulation can effectively remove the defective genes, allowing the body to produce healthy red blood cells and potentially curing the disease.
What ethical concerns arise from the use of CRISPR gene editing technology?
CRISPR gene editing raises several ethical questions, including the implications of altering human genes, equity in access to these treatments, and the potential for unintended consequences. Concerns also involve decisions related to genetic modifications that might enhance attributes rather than just heal medical conditions, prompting debates over human diversity and the nature of ‘normal’ health.
How does CRISPR gene editing relate to health equity?
CRISPR gene editing poses significant health equity challenges. High treatment costs, such as the $2.2 million for sickle cell therapy, can limit accessibility for marginalized populations. Consequently, innovations in gene editing might improve health only for those who can afford them, leaving vulnerable communities further behind, thus highlighting the need for equitable access to medical technology.
What is the significance of somatic versus germline CRISPR gene editing?
The distinction between somatic and germline CRISPR gene editing lies in the impact of genetic modifications. Somatic editing alters genes in non-reproductive cells, affecting only the individual treated, while germline editing modifies genes that can be inherited, impacting future generations. This raises profound ethical issues about the long-term effects and the implications of ‘designing’ offspring.
Can CRISPR gene editing unintentionally cause health issues?
Yes, CRISPR gene editing can have unintended consequences. While it aims to edit specific genes, the complexity of genetic interactions means alterations could inadvertently affect other genes, leading to unforeseen health problems. This emphasizes the importance of robust research and ethical oversight in gene editing applications.
How does CRISPR gene editing connect to discussions about medical technology?
CRISPR gene editing is at the forefront of medical technology, representing a transformative approach to treating genetic disorders and advancing personalized medicine. Its ability to efficiently modify genes holds great promise for future therapeutic innovations, but it also necessitates critical discussions about the ethical and societal implications of such powerful technology.
| Key Points | Details |
|---|---|
| Introduction to CRISPR | Discussion revolves around the ethical implications of modifying the human genome using CRISPR technology. |
| Neal Baer’s Experience | Baer shares challenges in treating conditions like sickle cell anemia and highlights the potential CRISPR has to cure these diseases, raising the question of whether we should. |
| Somatic vs. Germline Editing | CRISPR allows for editing both somatic cells (non-heritable) and germline cells (heritable), leading to permanently altered traits in future generations. |
| Ethical Dilemmas | Debate over which diseases warrant gene editing, and who decides the ethical boundaries of such modifications. |
| Cost and Accessibility | The average cost for CRISPR treatments (e.g., sickle cell) is $2.2 million, raising questions about health equity. |
| Innovation vs. Justice | Brendel emphasizes the importance of considering health justice and fairness when innovating gene editing. |
| Unintended Consequences | Potential for unforeseen effects from gene editing, as genes interact in complex ways within the body. |
| Global Oversight | Concerns over the enforcement of laws concerning gene editing in countries with less stringent regulations. |
Summary
CRISPR gene editing presents a revolutionary potential for addressing genetic diseases, yet it also raises significant ethical questions about the role of humanity in altering the very fabric of life. While the ability to cure conditions like sickle cell anemia is promising, we must critically assess the moral implications of such technologies. Balancing innovation with health equity and ensuring proper oversight is vital to navigate the complexities introduced by this powerful tool.